crsublette
coyotes call me Charles
I think too often simplistic answers are given which purposely leave holes in the flow of thought to create confusion and this leads to misinformation, which could lead to bad decision making that could be harmful to aquatic life.
When we are talking fundamentals, ponds are essentially just outdoor aquariums. The basic fundamentals are always constant. The only variables different between ponds and aquariums is the chemical synthesis when dosing the water, pond's greater surface area and evaporation, increased contaminants in ponds, and the materials and methodologies will vary according to the ecosystem's context. However, the basic fundamentals still remain the same.
One thing to note is that species and chemistry do change in saltwater ecosystems. So, you must keep this in mind when reading articles in aquaculture revolving around saltwater, which is marine, aquatic life. It is best to stick to the facets in the aquaculture arena relating to freshwater since our pond's aquatic life are freshwater in nature.
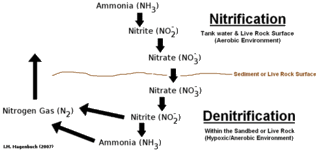
Out of my research library, here are a couple of articles that I have found to be the best in explaining the complete ecosystem.
To put it simplistic, autrotrophs are the "good guys" and heterotrophs are the "bad guys"; however, not all heterotrophs are "bad guys", just most of them are.
Autotrophic Nitrifying Bacteria and Their Practical Application in Freshwater Aquariums
Heterotrophic Bacteria and Their Practical Application in Freshwater Aquariums
However, I do disagree with how Dr. Hovanec and Tetra claim sole credit for this discovery and sole access to patents in regards to having a product that can jump start a pond's ecosystem.
For further research into the bacteria arena and products available out there, then check out the major leaders in the research arena involving bacteria in both freshwater and marine ecosystems. However, focus on the freshwater aspect to be applied to our ponds.
Keeton Industries
Bacta-Pur
Personally, I do not recommend the use of autrotrophic bacteria products when they are not refrigerated. Refrigeration greatly increases oxygen saturation which allows the autotrophs to remain in hibernation while preventing the heterotroph population from "crowding out" and consuming the autotrophs. Often times, if the product is not refregerated, then the product attempts to accumulate the dimorphic heterotroph "good guys", but this is an extremely fine line to walk due to how products can potentially be mishandled during transportation and storage in warehouses.
During my long days tractor "driving" (since everything is driven by GPS and computers nowadays), I have found this reading material makes my day much more enjoyable and shorter. One day I am going to have my research library annotated and compiled into a single reference guide that I hope to share and hope it will continually evolve as my knowledge grows, progression continues, and our understanding of the freshwater ecosystem grows.
By all means, feel free to contribute to ensure the accuracy and sharing the progressions to help increase our knowledge base of our pond's freshwater ecosystem.
When we are talking fundamentals, ponds are essentially just outdoor aquariums. The basic fundamentals are always constant. The only variables different between ponds and aquariums is the chemical synthesis when dosing the water, pond's greater surface area and evaporation, increased contaminants in ponds, and the materials and methodologies will vary according to the ecosystem's context. However, the basic fundamentals still remain the same.
One thing to note is that species and chemistry do change in saltwater ecosystems. So, you must keep this in mind when reading articles in aquaculture revolving around saltwater, which is marine, aquatic life. It is best to stick to the facets in the aquaculture arena relating to freshwater since our pond's aquatic life are freshwater in nature.

Out of my research library, here are a couple of articles that I have found to be the best in explaining the complete ecosystem.
To put it simplistic, autrotrophs are the "good guys" and heterotrophs are the "bad guys"; however, not all heterotrophs are "bad guys", just most of them are.
Autotrophic Nitrifying Bacteria and Their Practical Application in Freshwater Aquariums
Heterotrophic Bacteria and Their Practical Application in Freshwater Aquariums
However, I do disagree with how Dr. Hovanec and Tetra claim sole credit for this discovery and sole access to patents in regards to having a product that can jump start a pond's ecosystem.
For further research into the bacteria arena and products available out there, then check out the major leaders in the research arena involving bacteria in both freshwater and marine ecosystems. However, focus on the freshwater aspect to be applied to our ponds.
Keeton Industries
Bacta-Pur
Personally, I do not recommend the use of autrotrophic bacteria products when they are not refrigerated. Refrigeration greatly increases oxygen saturation which allows the autotrophs to remain in hibernation while preventing the heterotroph population from "crowding out" and consuming the autotrophs. Often times, if the product is not refregerated, then the product attempts to accumulate the dimorphic heterotroph "good guys", but this is an extremely fine line to walk due to how products can potentially be mishandled during transportation and storage in warehouses.
During my long days tractor "driving" (since everything is driven by GPS and computers nowadays), I have found this reading material makes my day much more enjoyable and shorter. One day I am going to have my research library annotated and compiled into a single reference guide that I hope to share and hope it will continually evolve as my knowledge grows, progression continues, and our understanding of the freshwater ecosystem grows.
By all means, feel free to contribute to ensure the accuracy and sharing the progressions to help increase our knowledge base of our pond's freshwater ecosystem.
